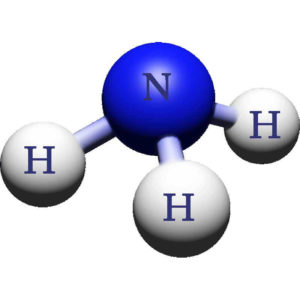
Ammonia is a compound consisting of one nitrogen atom and three hydrogen atoms and is denoted by the formula NH3. Its boiling point is -28°F at atmospheric pressure, so unless it is under pressure, it is gaseous at room temperatures. Therefore, pure ammonia is typically stored under pressure in a liquid form. Household ammonia is only 5-10% NH3, the remaining 90-95% is water. Ammonia is extremely soluble in water. It is often depicted like this:
In fact, ammonia’s affinity for water is so high, that industrial vent streams which might contain ammonia are often bubbled through vessels containing water, and the ammonia in the gas stream will safely dissolve into the water.
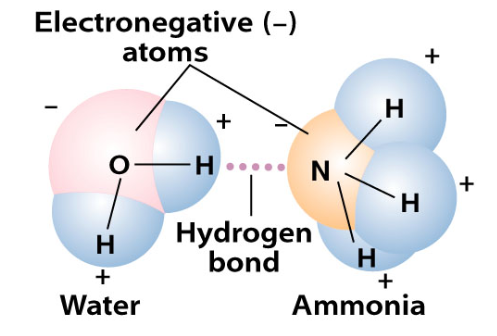
Why is this? The nitrogen atom is highly electronegative. It draws the electrons from the hydrogen atom to itself. Therefore, there is a slightly negative charge around the N and a slightly positive charge around the three H’s. This makes it a polar molecule. Water is also a polar molecule, with a negative charge around the O and a positive charge around its two H’s. Just like magnets, opposite charges attract each other. The H’s trying to get as close to either an O or an N as the molecules will allow. These are known as hydrogen bonds. Since these are both water and ammonia are small molecules, they can get pretty close to each other and form relatively strong hydrogen bonds.
Fun fact: water is much more strongly polar (oxygen is a stronger electronegative atom) than ammonia. This is why their boiling points are so different ( -28° for NH3 and 212°F for H2O). It takes that much more energy to break the hydrogen bonds of water….. Now you know!
Ammonia used in industry has had all the water removed from it and is called anhydrous ammonia. It is both toxic and flammable; its flammability concentration range is 15 – 28%. Ammonia has a strong distinctive odor that is easily detected by most people in concentrations as low as 20 parts per million (ppm). This is a good thing because the lethal concentration is 300 ppm, so any leaks, although dangerous, are usually detectable before becoming lethal.
Since the hazards of dealing with anhydrous ammonia are so serious, there are many references and guidelines available to lay out how to safely deal with it:
- The first place to look would be for any manufacturer’s Safety Data Sheet (SDS). Every SDS has sixteen sections which describe the compound’s physical characteristics, important reactivity considerations, etc. For example, anhydrous ammonia cannot be used with non-ferrous (e.g. copper) or galvanized metals
- The Occupational Safety and Health Administration created a standard just for anhydrous ammonia: OSHA 1910.11 – Storage and handling of anhydrous ammonia. It covers a multitude of aspects from storage vessel labeling to piping & pumping requirements
- The Environmental Protection Agency (EPA) has published the Accident Prevention And Response Manual For Anhydrous Ammonia Refrigeration System Operators, the title of which is pretty self-explanatory
- International Institute of Ammonia Refrigeration (IIAR) bulletins
- National Institute of Occupational Safety and Health (NIOSH) bulletins
In Part Two of this blog series, we’ll look at some of the major industrial uses for ammonia….
As President of The Warren Group, Jennifer Morningstar, PE, CFEI, has over 20 years of engineering experience. Her areas of emphasis include chemical release & exposure, OSHA compliance, boiler systems, industrial accident investigation, fires & explosions, product liability and scope of damage/cost to repair analyses. She spent 16 years working at a polyethylene terephthalate (PET) manufacturer. She is an OSHA-trained Process Hazard Analysis study leader and completed Root Cause Failure Analysis training to become an Incident Investigator. Jennifer authored procedures for lockout/tagout and confined space entry. She has experience as an energy management consultant in a variety of industries including mineral extraction, pulp & paper, animal harvesting & packaging (including rendering) and grain milling. Jennifer holds a Bachelor of Science Degree in Chemical Engineering from Virginia Polytechnic Institute and State University as well as a Master of Business Administration from the University of South Carolina.