Hidden Dangers at Home 3 Surprising Household Hazards You Shouldn’t Ignore
Most folks think that chemical hazards are strictly limited to manufacturing or other industrial sites. The fact that there are potential chemical hazards in the safe confines of their homes often comes as a surprise.
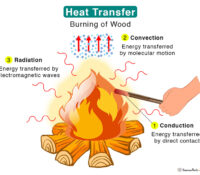
A surprising number of residential fires are caused by the spontaneous combustion of stain application materials. When an oil-based stain is applied to furniture or flooring, the oils react with the oxygen in the air to form very large polymer chains. The process of the liquid stain turning to solid barrier is called curing (it is not drying via evaporation) and the reaction is oxidation. This reaction generates heat. On the floor or chair, the heat dissipates into the room without an issue. That same oxidation reaction is occurring on the brush, towel, sponge, or rag that was used to apply the stain. If those materials are piled in a corner or in a trash can, the heat cannot dissipate. If there is enough product present, enough heat can be generated to Read More