Interpreting Industrial Incident Data – Lesson Learned
This is a case study about an incident I investigated involving a major upset in a distillation column. This blog builds on the previous blogs about the Distributed Control System, DCS – Data is the Key.
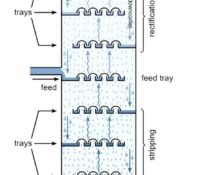
Distillation is a method of separating mixtures of compounds with differing boiling points. Uncle Bill with his still on the hill separates ethanol, that boils at 173°F, from water that boils at 212°F. If the mixture is heated to above 173°F, but below 212°F, the ethanol will boil, the vapor will travel up out of the unit and then can be condensed and served over ice with an olive… Any mixture of two or more chemicals with different boiling points can be separated in this way. The distillation Read More