Now that you know what ammonia is (see Part One here), how it behaves, and how to safely store it and work with it, let’s look at some areas in industry where it is used.
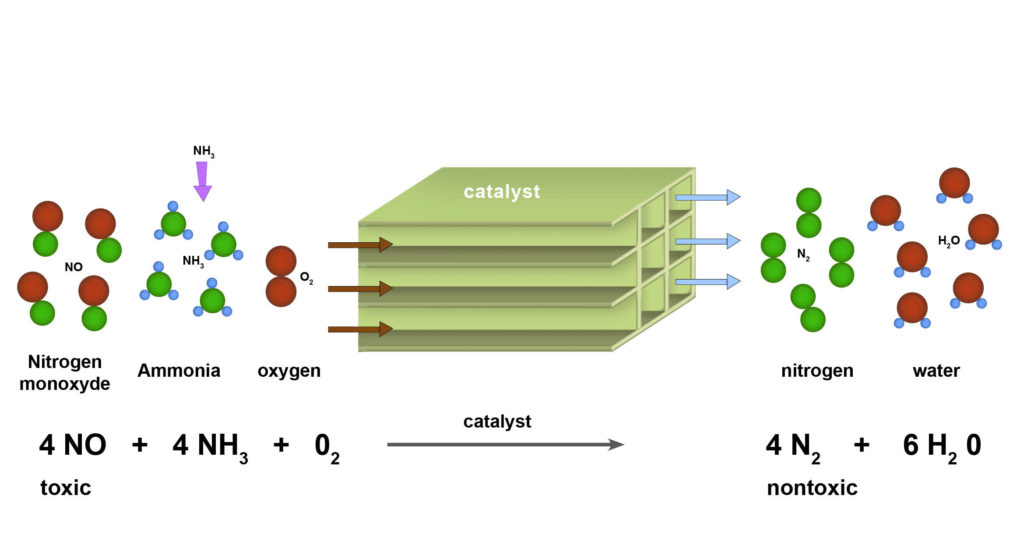
Anhydrous ammonia has a use in pollution control. Industrial boilers and power plants burn coal or natural gas to make steam and/or electricity. When the fuel is burned using air as the oxygen source nitrogen gets exposed to the heat as well because air is 79% nitrogen. The nitrogen gets oxidized and forms several compounds referred to as NOx (NO, NO2, NO3). NOx compounds are harmful to both the environment as the precursor to acid rain, and people as respiratory irritants. There are regulations in place that limit the amount of NOx that these power plants and other large combustion units can emit. Selective Catalytic Reduction is a technology that “unoxidizes” (or reduces, in chem-speak) by combining the vent gases with anhydrous ammonia (in the presence of a catalyst). In this process the NOx gets converted back to N2, harmless nitrogen.
Anhydrous ammonia is also used as a refrigerant in larger industrial/commercial installations. The safety issues with large amounts of ammonia used under pressure preclude its use in residential systems. It was one of the original refrigerants, along with butane, but both fell out of favor for being toxic/flammable when CFCs (chlorinated fluorocarbons) came on the scene. Being neither toxic nor flammable, CFCs were considered a ‘safer’ refrigerant. It was then later discovered that CFCs in the upper atmosphere may play a part in thinning of the ozone layer.
The American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE) published a position document (expired Feb 1, 2020) recommending ammonia as a refrigerant. There is also a group, International Institute of Ammonia Refrigeration (IIAR), which publishes bulletins on the use and handling of ammonia in use in this capacity.
Ammonia is also used as a fertilizer; directly injected into fields. The ammonia reacts with the water in the soil; you may remember from the previous blog that anhydrous ammonia has a very high affinity for water. As the ammonium ion, it is stable in the soil and in a form that plants can take up through their roots to form chlorophyll.
Anhydrous ammonia is the starting point for several intermediate chemicals that are, themselves, the building blocks for more complex compounds. This is a very short list of some of them:
- Nitric acid – used to manufacture explosives
- Ammonium Nitrate – made from nitric acid and is used as a fertilizer
- Acrylonitrile – polymers, specifically polyamides (Nylon is a type of polyamide)
- Anilines – synthetic rubbers and dyes
- Sulfonamides – “Sulfa” drugs; common pharmaceutical
This list could easily go for a couple of pages, but these five examples show how diverse the end uses of the chemicals that start with anhydrous ammonia can be. This proves the age-old adage “Don’t judge a compound by its smell”!
As President of The Warren Group, Jennifer Morningstar, PE, CFEI, has over 20 years of engineering experience. Her areas of emphasis include chemical release & exposure, OSHA compliance, boiler systems, industrial accident investigation, fires & explosions, product liability and scope of damage/cost to repair analyses. She spent 16 years working at a polyethylene terephthalate (PET) manufacturer. She is an OSHA-trained Process Hazard Analysis study leader and completed Root Cause Failure Analysis training to become an Incident Investigator. Jennifer authored procedures for lockout/tagout and confined space entry. She has experience as an energy management consultant in a variety of industries including mineral extraction, pulp & paper, animal harvesting & packaging (including rendering) and grain milling. Jennifer holds a Bachelor of Science Degree in Chemical Engineering from Virginia Polytechnic Institute and State University as well as a Master of Business Administration from the University of South Carolina.