Around Christmas day of 2022, a mass of very cold air settled in the deep south/southeastern United States (where I live). It remained below freezing for over 24 hours. That is very unusual down here. There was a spate of burst pipes all over the area. Why? Water is one of a very few compounds that expands when it freezes…by approximately 9%!! If a volume of water is sealed off in a section of piping, and that water freezes, it is likely the pipe will rupture. Then, when it warms up again and thaws, liquid water will leave the pipe for an adventure!!
Water expands when it freezes because the water molecules form a six sided crystalline structure in its solid form (remember those six sided snowflakes we all cut out in grade school?). These large crystals cannot nestle together as closely as individual water molecules. So frozen water needs more elbow room, so to speak. The other way to state this phenomenon is that ice is less dense than water. Which is a really good thing for all the fish that live in ponds where it gets cold enough to freeze! The layer of ice on top of a pond insulates the water beneath it from colder air temperatures. If that ice sunk to the bottom of the pond instead, the water on top would freeze then sink and eventually the entire pond could freeze and kill the fish in it.
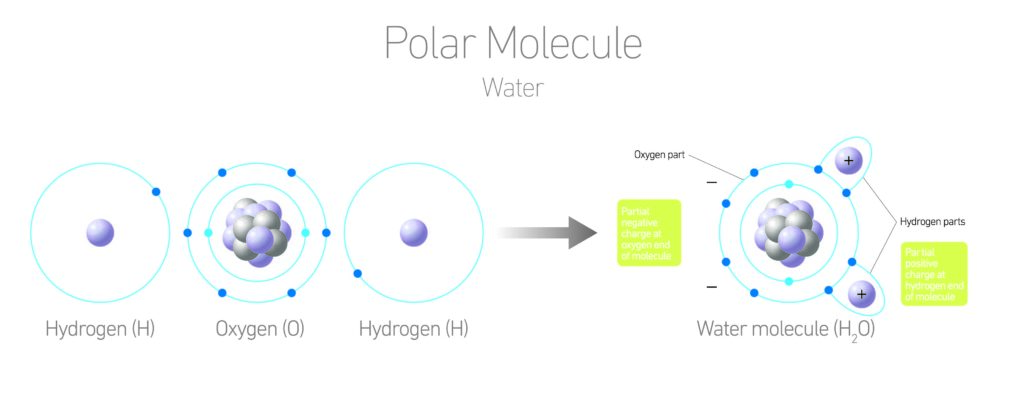
This made me start to think about all the other ways that water is unlike other liquids… and there are quite a few. They all stem from the fact that water has something called hydrogen bonds or polar bonds between its molecules. You all know that water has 2 hydrogen atoms and 1 oxygen atom: H2O. What most folks don’t know is that each hydrogen atom has one electron to donate to a bond and oxygen needs two electrons to fill its orbital shell. This configuration gives a slightly negative charge to the oxygen end of the molecule, and a slightly positive charge to the hydrogen ends. These charges act like little magnets and opposites attract!
These bonds give water properties that other compounds of similar molecular weight do not have…. Such as
- Relatively High Boiling point – Water has a molecular weight of 18 (O=16 , H=1). Ammonia (NH3) has a molecular weight of 17 (N=14, H=1). All other things being equal, they should have similar properties. But I’ve already told you that all other things aren’t equal, haven’t I?? Those hydrogen bonds require more energy to pull them away from one another. That means a higher boiling temperature. Ammonia boils at -28.01°F and water boils at 212°F. Quite the difference.
- High Surface Tension – surface tension is defined as “the property of the surface of a liquid that allows it to resist an external force.” Once again, due to the hydrogen bonds, water has the second highest surface tension of any liquid (only elemental Mercury has a higher value). This can be seen in water sheeting off of eaves during a heavy rain, or, back to our fish pond again, water striders doing their thing!
-
- Highest Specific Heat Capacity of Any Liquid- This means it takes a lot of energy to raise the temperature of a given volume water and it also means water can dissipate that heat throughout the entire volume easily. The best visual I’ve seen to explain this is placing a paper cup full of water into a campfire. As long as water is present, the paper won’t burn. Here’s a link to a video (you should really watch this!): https://www.youtube.com/watch?v=fpjLcLa7rkU
This capability is also why water is used in industrial sites for absorbing or transferring heat…there’s literally no better liquid for it. This ability to absorb heat without changing temperature is why the oceans don’t get as hot or as cold as the land… water takes a long time to heat or cool
Lastly, the volumetric ratio of steam to liquid water is higher than most other liquids….1700:1. In other words, if I had a 1ft x 1ft x 1ft cube of liquid water (1 cubic foot) that I turned it into steam and kept at atmospheric pressure, that steam would take up 1700 cubic feet of space. Comparing to ammonia, that ratio is 850.
In the same way that frozen water can rupture pipes, water vaporizing to steam can have devastating effects on systems that aren’t designed for it. If you need an investigation of a loss involving water, in any of its forms, please call our experienced engineering experts at Warren.
As President of The Warren Group, Jennifer Morningstar, B.S.Ch.E, P.E., CFEI, has over 20 years of engineering experience. A licensed professional engineer in several states and a NAFI Certified Fire and Explosion Investigator, she holds a Bachelor of Science Degree in Chemical Engineering from Virginia Polytechnic Institute and State University, as well as a Master of Business Administration from the University of South Carolina. Throughout her engineering career, Jennifer has conducted forensic investigations involving chemical release/exposure, OSHA process safety management, industrial accident investigation, equipment failures, fires & explosions, and scope of damage/cost to repair. Jennifer is a member of the National Association of Fire Investigators (NAFI), the South Carolina chapter of the International Association of Arson Investigators (SCIAAI), and the American Institute of Chemical Engineers (AIChE).