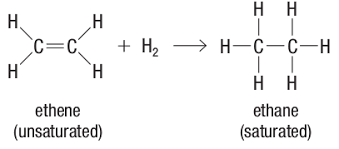In the old-timey Fire Triangle, you have heat, fuel, and oxygen. Get these three together in the right quantities, and you get fire. What if the fuel provides its own heat? That’s spontaneous combustion, or spontaneous ignition. NFPA921 defines this as “initiation of combustion of a material by an internal chemical or biological reaction that has produced sufficient heat to ignite the material.”
We’ll start with the self-heating. Exothermic reactions are chemical reactions that give off heat. For spontaneous combustion phenomena, the compounds seen are typically unsaturated hydrocarbons, for example; linseed oil, cooking oils, or lard. These materials must be in contact with combustible material with high surface area, such as rags, cardboard or sawdust. Unsaturated means that some of the carbon atoms in the chain are bonded to fewer than four other atoms. In other words, there is a double bond present.
When a chemical reaction breaks the double bond (mostly via interaction with air) some heat is generated. If the heat is allowed to dissipate, it is not problematic. But if the heat is trapped, it will build up and raise the temperature in the nearby area. The configuration of the combustible material is what either traps the heat or allows it to escape. Fire investigators at The Warren Group have been assigned fire losses where the causes were:
- Piled rags at a home remodel
- Piled towels from a restaurant at a dry cleaner
- Improperly stored rags at a painting facility
- Unemptied lint traps at a commercial laundry
- Unemptied bag on a floor sander
Another type of self-heating method is biologic in nature. In grain or hay storage, the respiration of bacteria, molds, and other microorganisms generate heat. If the heat can be drawn away from the grain, the temperature of the mass will not rise to dangerous levels. One method of removing heat from grain is aeration. This is the process of passing ambient air through stored grain. There are many different ways to aerate stored grain: top-down, bottom-up, positive pressure, negative pressure. The best option is dependent on the size and shape of the storage vessel, and type of grain being stored. The Warren Group has investigated a fire losses at grain storage facilities in which spontaneous combustion was the cause.
Once the self-heating is underway, all that remains is to allow enough time for the combustible material nearby to reach its autoignition temperature and the fire will commence. This is why many of these fires happen in the wee hours of the morning when a business is closed. When people are around, items get moved and heat gets dissipated.
So if you are using solvents, coatings, varnishes, paints…. You know, anything unsaturated, be sure to follow the manufacturers’ instructions for proper disposal and/or storage of brushes, rags, pads, etc. As a fire investigator, that is always something we track down.
Fun fact: hypergolic combustion is also spontaneous, but we rarely see it in the property loss world, which is a shame because it’s fun to say out loud. This is the spontaneous combustion that occurs when two separate components come in contact with each other. It is the main reaction behind launching a rocket into space!
Rock on…
As President of The Warren Group, Jennifer Morningstar, B.S.Ch.E, P.E., CFEI, has over 20 years of engineering experience. A licensed professional engineer in several states and a NAFI Certified Fire and Explosion Investigator, she holds a Bachelor of Science Degree in Chemical Engineering from Virginia Polytechnic Institute and State University, as well as a Master of Business Administration from the University of South Carolina. Over her 20-year engineering career, Jennifer has conducted forensic investigations involving chemical release/exposure, OSHA process safety management, industrial accident investigation, equipment failures, fires & explosions, and scope of damage/cost to repair. Jennifer is a member of the National Association of Fire Investigators (NAFI), the South Carolina chapter of the International Association of Arson Investigators (SCIAAI), and the American Institute of Chemical Engineers (AIChE).